Published: 11/2023
For traditional biomedical and human subjects research, investigators must obtain approval from their Institutional Review Board (IRB) prior to conducting a study. IRB evaluation and approval is essential to ensure research studies are conducted in an ethical manner and human subjects are protected. In practice, the IRB process can act as a significant barrier due to the amount of administrative work and advanced planning required.
Quality Improvement (QI) projects are meant to be rapid cycle improvements of the clinical environment. Often, these projects do not need IRB approval and are considered IRB exempt. First, it is important to determine if your QI project is research. Research is defined as “…a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge…” If your QI project does not meet this definition, it is not considered human subjects research and does not need to go through the IRB process. This is illustrated in Example 1.
Example 1: A clinic would like to ensure their patients are filling out an Edinburgh Postnatal Depression Scale at their 28-week visit. The clinic staff performs an audit to see what percentage of patients filled out the EPDS in that timeframe. They then perform an in-service regarding the importance of screen as an intervention to increase compliance.
If a QI project is considered to be research, many fall under IRB exempt status. This requires the collection or study of preexisting data sets, documents, records, or specimens, but only if these sources are publicly available or if the information is recorded by the investigator in such a manner that subjects cannot be identified, either directly or through identifiers linked to subjects [i.e., through use of a key]. If the research team does not receive, view or handle identifiable original source data at any point, the study may be “not human subject research.” This is illustrated in Example 2 below.
Example 2: A resident would like to look at the rate of surgical site infections (SSI) in emergent cesarean delivery at their institution. They will be using a de-identified birth complications database that the department uses for quality assurance purposes. They hope to identify the rate of SSI and implement a checklist to ensure infection prevention processes are maintained even in emergencies. This project would not be considered human subjects research and therefore does not need IRB approval or exemption.
There are some QI projects that will need IRB approval as they are considered research on human subjects (Example 3).
Example 3: The same resident in Example 2 now wants to prospectively trial a new abdominal prep to be used in emergent cesarean deliveries. Given this data will be prospectively collected and involves interaction with an individual it is considered human subject research and requires IRB submission and approval.
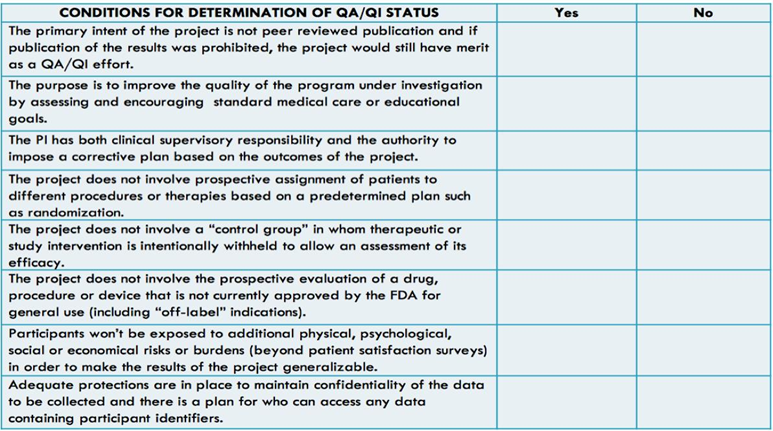
IRB interactions for QI projects can seem complex, therefore, some institutions have engaged with their IRBs to create checklists to assess if a QI project needs to go through the IRB process or not. If all criteria on the checklist have been met, a project is designated QI. One example of an institutional checklist is demonstrated in Figure 1.
Most QI projects are initiated with the intent to improve systems at the home institution. In the event that any outcomes are deemed worthy of dissemination, and thus presentation or publication, they will still fall under QI IRB status and can be published.
Figure 1: Example of a Quality Assurance/Quality Improvement (QA/QI) Checklist.
Note: If all responses are ‘‘Yes,’’ the project is approved as QA/QI status. If any response is ‘‘No,’’ the project must be submitted to the Institutional Review Board for approval.
Additional Resource
The US Department of Health and Human Services: Quality Improvement Activities FAQs. Accessed 10/23/23. https://www.hhs.gov/ohrp/regulations-and-policy/guidance/faq/quality-improvementactivities/index.html.