Published: 04/2025
Most quality improvement (QI) projects are initiated with the intent to improve systems at the home institution. When projects could help other institutions, it is important to consider submitting them for presentation or publication. In this case, it is important to remember several principles of publishing QI work.
First, you must determine if IRB approval is required for the project. Often, these projects do not need IRB approval and are considered IRB exempt. Please refer to our SASGOG Lexicon on this topic for more details.
Next, remember that QI work differs from traditional research. For reports on system level work in quality and safety, the SQUIRE (Standards for QUality Improvement Reporting Excellence) guidelines1 should be followed. The Introduction section must emphasize why your QI problem is relevant beyond your institution. This includes showing the general gap between current and preferred practice. The introduction must also end in a clear AIM statement.2
Example 1: An institution, combating an increasing surgical site infection (SSI) rate, found that instituting a specific method of abdominal preparation for urgent cesarean deliveries significantly reduced their SSI rate. The authors anticipate other institutions are facing similar challenges. Their AIM statement expresses they reduced the SSI rate by 50% in 3 months using the new preparation workflow.
Within the Methods section, state the context in which the work was performed. This may include the size, location, patient population, and resources available at the institution. Context is key! For QI work, the context helps the reader consider how a particular intervention can be enacted at their institution. This section will also have a detailed description of the implementation strategy, including outcome, process, and balancing measures.
Example 2: A health system hopes to increase their rates of breast cancer screening. They are tracking the percentage of eligible patients who are screened (outcome measure). Additionally, the number of patients who received counseling for breast cancer screening were monitored (process measure). They also recorded the time it took for counseling, ability to schedule appointments for screening, and changes in other cancer screenings (balancing measures).
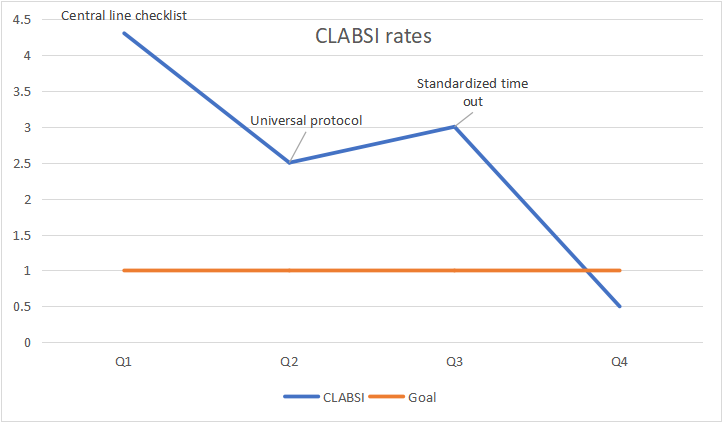
When reporting Results, include all your measures with a particular focus on the outcomes as this was your goal. Results should be reported using standard QI data reporting tools such as Pareto charts or Run charts. A Run chart is a graphic representation of your primary outcome over time. Figure shows a Run chart displaying a clinic’s central line-associated blood stream infection (CLABSI) rates over three consecutive PDSA cycles.
Figure 2: Central Line-Associated Blood Stream Infection Rates Over Time
Lastly, in the Discussion section, summarize your findings in the context of prior published work. This summary should include reflections on the implications of the project and lessons learned. Limitations for generalization should be clear. Anticipated next steps, including those for sustaining gains, should also be included.
References
- “Revised Standards for Quality Improvement Reporting Excellence. Accessed 7 April 2025. https://www.squire-statement.org. Accessed 7 April 2025.
- Institute for Health Care Improvement. “The Model for Improvement: Setting Aims.” https://www.ihi.org/how-improve-model-improvement-setting-aims.
Accessed 7 April 2025.
Further Reading
Wong and Sullivan. How to Write Up Your Quality Improvement Initiatives for Publication. JGME. 2016; May 1:128-133